What is pH?
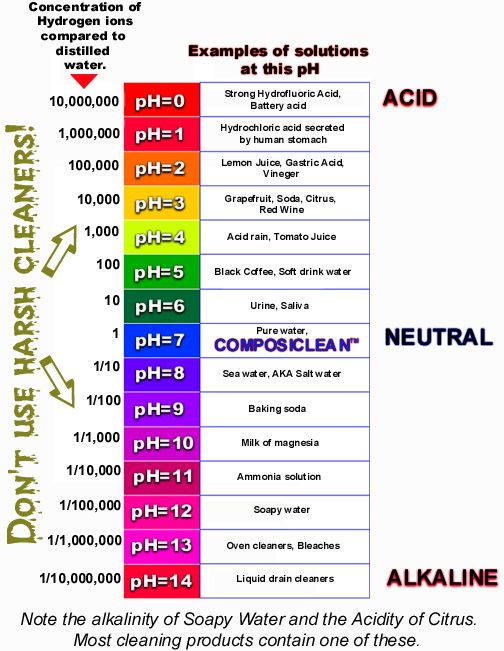
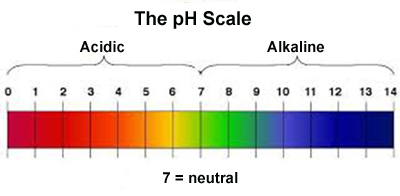
pH tells us how many ions or electrically charged particles are present in a liquid. The pH scale ranges from 0 to 14. Numbers below 7 are acid, while those greater than 7 are alkaline. The further from 7 the stronger the acid or alkaline. Each whole number difference represents a tenfold change.
Pure water is neutral - pH 7. However, almost anything dissolved in the water, in any amount, will affect the pH. Freshly distilled water rapidly absorbs carbon dioxide from the air and reaches a pH of 5.5 in a very short time. Other materials dissolved in the water may have an even more marked effect. Depending on its chemical characteristics, it can either raise or lower the pH value. Thus the pH of any solution depends on the type and amount of materials dissolved in the water.
Each number on the pH scale actually represents a 10 fold difference. Thus, pH 4 is ten times as acidic as a pH 5 solution. A pH 3 solution, in turn has ten times the strength of the pH 4 solution - and 100 times the acidity of the pH 5 solution. This pH scale of 0 to 14 depends on the chemical properties of water and this pH scale cannot be used without water.
Total alkalinity indicates how well a chemical compound will maintain its pH.
Buffers tend to resist any change in pH. If something tries to change the pH, how much strength is in reserve to maintain the ranks? Some materials tend to stabilize a solution so that it maintains a specific pH.
Ingredients in a cleaning product that increase the total alkalinity are called builders. Builders may be added to buffer a product. They also help to counteract the effects of hard water.
The natural pH of most carpet fibers is near neutral (7). For wool, the natural pH will be between 5.5 and 7. For the life of the fiber and to keep from attracting soils, we should leave the fibers as close as possible to their natural pH when the cleaning is finished.
Now that we all know what pH is, here is how it can be applied to carpet cleaning...
Most soils are acidic, averaging between 4 and 5. Soils will be easier to remove when they are neutral. When alkaline mixes with fats, oils, and grease, they form a soap.
The most effective cleaning agents are alkaline. Cleaning agents with higher pH can be used on olefin.
The pH factor can also be important in removing stains. It can be checked with paper strips (Phydrion paper).

To use pH paper, moisten the stain slightly with water and press a piece of the paper to the carpet and check its color on the pH chart provided.
